First EPITARGET Results
Work performed since the beginning of the project and the main results achieved so far
During the first year of EPITARGET, several deliverables and milestones have been achieved, all according to the project plans defined by the consortium.
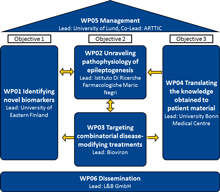
Objective 1:
Establishment of Epilepsy Preclinical Biomarker Bank (EPBB). To identify combinatorial biomarkers for epileptogenesis, EPITARGET will establish an Epilepsy Preclinical Biomarker Bank (EPBB) and respective database. EPITARGET has now developed Common Data Elements (CDEs), which are an integral part of Case Report Forms (CRFs) also developed by EPITARGET. Moreover, EPITARGET has now established and is in the process of test-driving common platform for database, REDCap, licensed to EPITARGET through the Coordinating Center ULUND, and the actual data are being included.
Collection of samples from animal experiments for identifying biomarkers for epileptogenesis. An important part of biomarker identification in EPITARGET is the collection of samples from animals. The collection of various samples for different modalities of potential biomarkers is well on its way. These samples are being collected by EPITARGET centers according to the CRFs and the CDEs, in various models of epileptogenesis. Moreover, EPITARGET has reached an important technological achievement by developing biocompatible electrodes for fast and sensitive glucose measurement in the brain.
Objective 2:
Brain expression, blood levels and neuromodulatory properties of molecules involved in key etiopathogenic mechanisms of epileptogenesis. To be able to counteract several mechanisms involved in epileptogenesis, it is crucial to identify and explore temporal and spatial expression of the target molecules. Therefore, one of the main priorities in the first reporting period of EPITARGET was to identify those key factors and explore their expression after epileptogenic insults. EPITARGET made a substantial progress, and have now characterised expression patterns of a number of molecules and factors at various timepoints after epileptogeneic insults. These factors include proresolving lipid mediators and their receptors, microRNAs, immunoproteasomes, free radicals and antioxidants, amyloidogenic factors, inflammatory molecules, extracellular matrix proteins, and synaptic proteins.
As part of the combinatorial treatment strategies, EPITARGET has explored pharmacokinetics and tolerability of combinations of clinically available drugs in naive and epileptic rodents. The tolerability of drug combinations were explored and based on an algorithm developed by EPITARGET, whereby combinations of 2-3 drugs were tested at different doses in naive rodents and, if tolerable, in epileptic rodents.
Several achievements have also been reached in refining the SE models, predicting epilepsy development after SE by PTZ and behavioural excitability tests, and optimization of two-stage approach for combination drug screening, all to harmonize and optimize experimental models across the Consortium.
For targeting combinatorial disease-modifying treatments into the brain, EPITARGET has developed lipid encapsulation for molecules, the first formulation of Amiloride being validated in vitro and ready for in vivo testing. First generation Amplicon viral vectors for long-term transgene expression have been verified in in vivo studies.
Objective 3:
Translating the knowledge obtained in animal models to patient material. EPITARGET has successfully established procedures instrumental for the initiation of collection of post mortem human brain tissue samples from patients after status epilepticus (SE) and traumatic brain injury (TBI), surgical brain tissue and biofluids (CSF and blood) from patients after TBI, and fresh-frozen hippocampal biopsy specimens from pharmacoresistant epilepsy patients, as well as blood from a subset of these patients. The sample collection is currently ongoing.
The expected final results and their potential impact and use
The overall expected final results of EPITARGET are to (i) identify combinatorial biomarkers for epileptogenesis, diagnostics, prediction of disease progression and pharmacoresistance; (i) identify combinatorial preventive and disease-modifying treatments targeting complex pathophysiological mechanisms of post-insult epileptogenesis; and (iii) validate combinatorial biomarkers from animal studies in human subjects and tissue. These results are envisaged to have a dramatic impact on the socio-economic burden of epilepsy by improving quality of life for millions of people and their families, reducing the costs related to epilepsy, currently estimated as 20 billion € for Europe only. Societal implications of the final results of EPITARGET are expected to have significant influence on a wide range of areas including patient and professional organizations, professionals in clinical practice, regulatory authorities, pharmacological industries and SMEs, funding organizations, other brain diseases, as well as on health status of the general population.
Objective 1:
Establishing Epilepsy Preclinical Biomarker Bank (EPBB) and database will have an impact not only on the outcomes of EPITARGET, but will have wider implications for the epilepsy research community, as well as for other areas of neurological diseases. This will be one of the first animal biobanks and databases that will lay a foundation for other disease model biobanks, facilitating the preclinical research. CDEs and CRFs already developed by EPITARGET will be valuable for ILAE task force dedicated for this mission, as well as for other consortia in epilepsy established in USA by NINDS initiative for Centers Without Walls (CWOW), such as EpiBios. EPITARGET CDEs will be available to the general public and research communities on the EPITARGET webpage.
Objective 2:
EPITARGET has already generated data on temporal and spatial patterns of gene and protein expression for a wide range of factors and molecules potentially involved in epileptogenesis, which is broadening our understanding of epileptogenesis, and eventually will result in developing combinatorial antiepileptogenic treatment strategies.
Results obtained during first year of EPITARGET in evaluating combinatorial treatment approaches based on existing drugs will have an impact on shifting the paradigm of thinking about possibilities for preventive and disease modifying treatment strategies after brain insults. Novel drug combinations are also generating results and will increase our knowledge and understanding of antiepileptogenic treatment possibilities. Moreover, results on optimization and standardization of post-SE animal models are very useful not only for EPITARGET but the whole epilepsy research community, and will contribute for more understanding of those factors, such as animal strain and batch variability, SE duration and termination procedures, site of insults of the brain, etc., in contributing variable outcomes of the preclinical studies.
Large capacity Amplicon viral vectors and lipid encapsulation approaches that are validated by EPITARGET will open novel avenues for combinatorial treatment approaches in preventing epileptogenesis and disease progression, and contribute to innovative thinking and exploitation of the results for commercialization strategies of SMEs.
Objective 3:
Prospective collection of samples from brain tissue, imaging, EEG and fluids from TBI and epilepsy patients will have a crucial role in securing validation of animal data in human material, paving foundation for translational research and clinical applications. Inclusion of patient data, and establishment of sample collection logistical details secures timely progress of validation process envisaged by EPITARGET. This material can also be used for data mining and systems biology approaches, and may also lead to human biobank development in the future, which will have significant impact on the research possibilities even outside EPITARGET.